Water Dynamics in polymeric systeMs
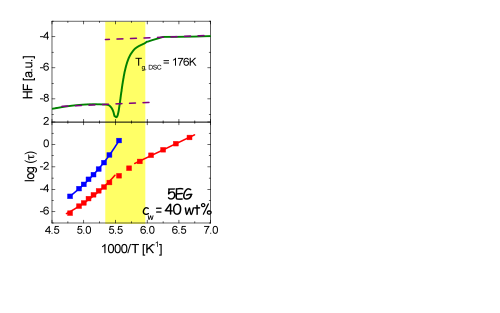
1) A crossover from non-Arrhenius behaviour at higher temperatures to Arrhenius temperature dependence at low temperatures was founded at a temperature close to the calorimetric glass transition (Tg,DSC)
Universal Behaviour of polymeric water solutions
The study of water dynamics in solutions of polymeric materials open a route to understand water dynamics in more simple environments than a bio-system.
NMR of Poly(vinyl pyrrolidone)-water solutions
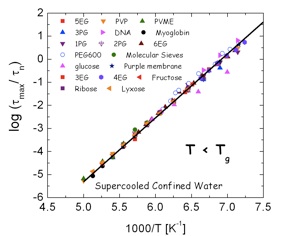
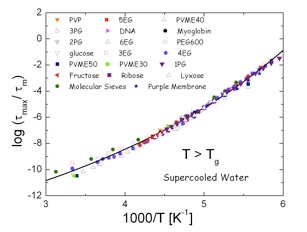
Master curve of the dielectric relaxation time for water dynamics in a wide variety of systems at temperature lower (A) and higher (B) than Tg,DSC.
Biological systems: myoglobin (44 wt%), DNA (30wt%) Sugars: fructose (30wt%), lyxose (30wt%), ribose (30wt%) Polymeric systems: PVME (poly(vinyl methyl ether) (40 and 50 wt%) and PVP (poly(vinyl pyrrolidone) (30, 40 and 55wt%). Other synthetic systems: sorbitol (40wt%), 3EG (triethylene glycol), 4EG (tetraethylene glycol), 5EG (pentaethylene glycol), 6EG (hexaethylene glycol) (35wt%), nPG (n = 1,2,3, propylene glycol, dipropylene glycol (2PG) and tripropylene glycol (3PG) (40 and 50wt%), PEG600 (Poly(ethylene glycol), Mn = 600g/mol) (33 wt%). In addition we also show the results for water geometrically confined in molecular sieves and purple membrane
By studying water dynamics in a collection of hydrophilic aqueous solutions (synthetic polymers, small organic molecules and bio-polymers) in the low temperature range 130-250K we found some typical features of water dynamics in solutions:
Thus, below Tg,DSC of the system, water is restricted to move in confined spaces, whereas above Tg of the system the water molecules are able to perform cooperative long-range motions.
2) We have associated the crossover with the emergence of confinement effects due to the freezing of the host material.
-
3)We also found a universal behaviour for the temperature dependence of the relaxation times both above and below the calorimetric glass transition temperature (Tg,DSC) as seen in the Figures below.
-
4)Below Tg,DSC an almost constant value of the activation energy Ea= 0.54eV was founded for high water concentration.
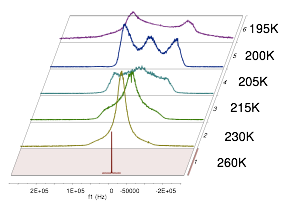
Neutron Scattering of PVME-water solutions
We are using nuclear Magnetic Resonance (NMR) spectroscopy to study the mechanisms for the reorientation of water in a solution with poly(vinyl pyrrolidone).
Representative papers:
Universal features of water dynamics in solutions of hydrophilic polymers, biopolymers, and small glass-forming materials Silvina Cerveny, Angel Alegría, and Juan Colmenero - PHYSICAL REVIEW E 77, 031803 (2008)
Broadband dielectric investigation on poly„vinyl pyrrolidone and its water mixtures Silvina Cerveny, Angel Alegría, and Juan Colmenero.THE JOURNAL OF CHEMICAL PHYSICS 128, 044901 (2008)
Dielectric relaxations in ribose and deoxyribose supercooled water solutions. S. E. Pagnotta, Silvina Cerveny, Angel Alegría, and Juan Colmenero. THE JOURNAL OF CHEMICAL PHYSICS 131, 085102 (2009)
FALTA COMPLETAR